By: Ranier Simons, ADAP Blog Guest Contributor
The primary weapons in the fight against HIV are anti-retroviral drugs, commonly referred to as ARVs. ARVs work by lowering the levels of HIV, known as viral load, in the body to stop HIV’s damage to the immune system. Viral load is measured by the number of copies of HIV RNA in a milliliter of blood.[1] The goal of ARV’s is to decrease the viral load to the point that standard tests cannot detect it. This level of viral suppression is commonly known as ‘undetectable’, and with it comes the important 'undetectable equals untransmissible' (#UequalsU) treatment goal.[2] Moreover, viral suppression means a significant reduction in the damage HIV can do to the body. This fight recently gained a new weapon, with two-month dosing of the injectable HIV therapy, Cabenuva.
Effective treatment means maintaining the levels of the medications in the body. This requires adherence to daily dosing. Not keeping the drugs at appropriate levels in the blood can increase viral load, damage immune cells such as CD4’s, and even drug resistance.[3] Drug resistance means that mutations of HIV could develop that are not responsive to a person's treatment regimen rendering that regimen ineffective and making it harder to find other medications that will work.
 |
| Photo Source: Very Well Health |
Early in the fight against HIV, drug regimens consisted of multiple pills several times a day. Toxicity issues, as well as issues keeping dosage schedules, were significant barriers to treatment. Modern-day once-daily one pill regimens were developed to combat both of those issues. However, some patients still experience difficulties with adherence. The most reported adherence challenges are forgetfulness, stigma, health perception, and digestive side effects.[4]
Some patients report having issues remembering to take their medication daily, especially those who have been on long-term therapy. Stigma is a concern by those who do not want family members to know they are living with HIV or to see them take medications. They do not want coworkers to know they are on medication and are psychologically harmed by the daily reminder of their health status. Health perception is an issue for long-term ARV patients because they feel healthy; thus, they become inconsistent with their dosing. Some patients also are inconsistent with their regimens due to perceived and experienced side-effects they experience from the drugs.
The evolution of ARV drug development has led to recent innovations to battle adherence issues. In January 2021, the U.S. Food & Drug Administration (FDA) approved a once-a-month injectable HIV treatment called Cabenuva. It is an extended-release injectable of cabotegravir and rilpivirine. The drug is a collaboration between Janssen Pharmaceuticals of Johnson & Johnson and ViiV Healthcare. Cabenuva is two separate injections into the buttock muscles, with the extended-release rilpivirine having been developed by Janssen and the extended-release cabotegravir from ViiV Healthcare.[5]
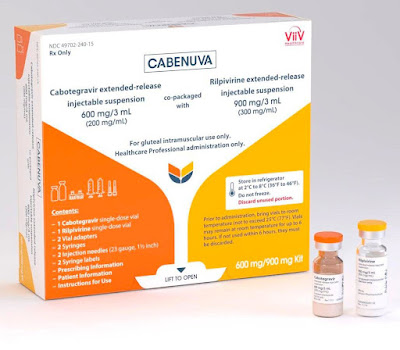 |
| Photo Source: The New York Times |
Cabenuva is meant for those who are virologically suppressed on a stable regimen and who have no history of treatment failure or known resistance to cabotegravir or rilpivirine. Proper administration requires one month of daily administration of rilpivirine and cabotegravir in pill form to assess tolerance before beginning the monthly injections of Cabenuva.
Continued research into daily Cabenuva proved that it is effective. Its efficacy has proven to be so positive that on February 1, 2022, the FDA approved it for two-month injectable treatment. Therefore, some patients can be treated with only six sets of injections annually instead of twelve. The extended label approval of Cabenuva from one to two months is based on the ATLAS-2M phase IIIb trial results. An ongoing clinical trial shows that in terms of virologic suppression, every two-month dosing is not inferior to monthly dosing. ATLAS-2M is being conducted in Australia, Argentina, Canada, France, Germany, Italy, Mexico, Russia, South Africa, South Korea, Spain, Sweden, and the United States.[6]
The most common adverse reactions were fever, injection site reactions, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The type and frequency of experienced adverse reactions were similar in those receiving the injection monthly or every two months. Additionally, the rates of serious adverse events or withdrawal due to adverse events were similar between the two groups.
This new FDA approval has the potential to be life-changing for those where Cabenuva treatment is appropriate. For further information on ATLAS-2M, please see https://clinicaltrials.gov/ct2/show/NCT03299049.
[1] Peabody, R. (2017, May). Viral load. Retrieved from https://www.aidsmap.com/about-hiv/viral-load#:~:text=The%20results%20of%20a%20viral,100%2C000%20would%20be%20considered%20high
[2] NIH. (2020, June 12). 10 Things to know about HIV suppression. Retrieved from https://www.niaid.nih.gov/diseases-conditions/10-things-know-about-hiv-suppression#:~:text=Daily%20antiretroviral%20therapy%20can%20reduce,to%20keep%20the%20virus%20suppressed
[3] Peabody, R. (2019, July). Adherence to HIV treatment. Retrieved from https://www.aidsmap.com/about-hiv/adherence-hiv-treatment
[4] Genberg, B. L., Lee, Y., Rogers, W. H., & Wilson, I. B. (2015). Four types of barriers to adherence of antiretroviral therapy are associated with decreased adherence over time. AIDS and behavior, 19(1), 85–92. https://doi.org/10.1007/s10461-014-0775-2
[5] Janssen Pharmaceuticals. (2022, February 1). U.S. FDA approves CABENUVA (rilpivirine and cabotegravir) for use every two months, expanding the label of the first and only long-acting HIV treatment. Retrieved from https://www.janssen.com/us/sites/www_janssen_com_usa/files/us_fda_approves_cabenuva_rilpivirine_and_cabotegravir_for_use_every_two_months_expanding_the_label_of_the_first_and_only_long_acting_hiv_treatment.pdf
[6] ViiV Healthcare. (2022, February 1). ViiV Healthcare announces us FDA approval of Cabenuva (cabotegravir, rilpivirine) for use every two months, expanding the label of the first and only complete long-acting hiv treatment. Retrieved from https://viivhealthcare.com/en-us/media-center/news/press-releases/2022/january/viiv-healthcare-announces-us-fda-approval-of-cabenuva/
Disclaimer: Guest blogs do not necessarily reflect the views of the ADAP Advocacy Association, but rather they provide a neutral platform whereby the author serves to promote open, honest discussion about public health-related issues and updates.

No comments:
Post a Comment