By: Brandon M. Macsata, CEO, ADAP Advocacy Association
****Important Support Resources Included****
Recently, I posted some commentary on my personal Twitter handle (@Purple_Strategy) noting an observed uptick in HIV stigma in the gay dating world. Gay dating apps are notorious for it. Likely, it isn't limited to the dating space, evidenced by a recent report issued by GLAAD, as well as subsequent commentary in private conversations and social shares highlighting examples of HIV stigma. It is very rare for me to interject my personal life's situations into ADAP Advocacy's daily advocacy and public policy activities, but it is vitally important to combat such stigma whenever possible. Frankly, HIV stigma has no place in my life and thus it needs to stay in its lane.
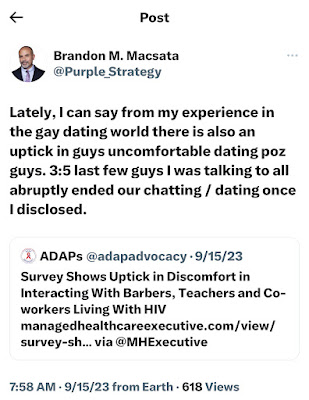 |
I'm a Taurus; we're pretty confident. I'm Italian; we're tough as nails. You hit me; I hit you ten times harder. But not everyone is like me. These recent dating rejections surrounding my status (undetectable, since 2004) weren't the first experiences with HIV stigma, and I know they won't be the last of them. But I can honestly say that I've taken them with a grain of salt. And now, U=U (undetectable equals untrabnsmittable) and fine work being done by Prevention Access Campaign and U=U plus has changed the national conversation.
BUT! Not everyone is a stubborn bull like me. I've had countless conversations with friends and colleagues, whereby their personal "run-ins" with HIV stigma really hurt them. It hurt their feelings, self-confidence, pride, and dare I even say, their self-worth. It has always bothered me on a very deep level seeing them struggle to cope with the ugliness that is HIV stigma.
Fighting HIV stigma won't come easily. According to GLAAD's 2023 State of HIV Stigma Report, only half of the respondents indicated they're "knowledgable" about HIV. Whereas GenX is considered most "knowledgable" about HIV, still one in four don't fall into that designation. What is most troubling is the trend line is going in the wrong direction on the general publics' comfortability interacting with people living with HIV—especially among certain professionals such as barbers or hair stylists, and teachers. Interacting with co-workers living with HIV is now problematic for 1:3 respondents.
 |
| Photo Source: GLAAD, 2023 |
It is 2023, and we're still dealing with 1993 attitudes (pre-HAART). I truly believe that there is plenty of fight left in all of us. I also believe that we are in this fight together, so I felt compelled to share some helpful resources available to my fellow POZ folks who might be coping with HIV stigma:
- Prevention Access Campaign - U=U Resource Center
- CDC - Facts About HIV Stigma and Discrimination
- CDC - Ways to Stop HIV Stigma and Discrimination
- CDC - Stigma Scenarios: Support in Action
- HIV.gov - Standing Up to Stigma
- GLAAD - 2023 State of HIV Stigma Report
- Healthline - How We Start Erasing the Stigma Around HIV
- Center for HIV Law & Policy - Stigma
- SERO Project - United States Resources and Groups
- Transgender Law Center - Legal Information Helpdesk
- Positively Aware - A Day with HIV
- Greater than HIV - Real People. Real Stories.
- ImstillJosh - Twitter
- MarninaTheQueen - TikTok
- PozLeigh - YouTube Channel
(email info@adapadvocacy.org if you wish to recommend a resource be added above)
Life is hard enough without having to confront stigma, simply over sero status. HIV stigma says more about the people dishing it out, and less about defining who you are. We have the tools to keep HIV stigma in its lane. There are over a million of us POZ folks in the United States, so remember that you're not alone and there are resources available!