By: Brandon M. Macsata, CEO, ADAP Advocacy Association
For over a decade counterfeit drugs have been a concern of the ADAP Advocacy Association, especially with all of the hoopla over drug importation. Despite the unsubstantiated claims about lowering drug costs for Americans, drug importation would come at too high a cost for people living with chronic health conditions, including HIV. Such an ill-advised public policy change would weaken the safety of the nation's drug supply chain, and prescription anti-retroviral (ARVs) medications aren't immune from counterfeit threats. The recent news about fake Symtuza® – prescribed medicine for the treatment of HIV-1 infection – identified in the United States is a case in point about looming risks.
 |
| Photo Source: MPR |
In December 2020, Janssen Pharmaceutical Companies of Johnson & Johnson issued a drug warning alert about the counterfeit HIV drug. As you can read here, Janssen was made aware that counterfeit Symtuza® had been distributed to three pharmacies in the United States.
Janssen's alert, in part, read:
"We are working closely with the U.S. Food and Drug Administration (FDA) to prevent further distribution and to support the agency’s investigation into the reported instances. The pharmacies involved procured the counterfeit product from distributors that have not been authorized by Janssen. We are confident that SYMTUZA® obtained through authorized distributors is authentic and safe for use, in accordance with the Prescribing Information."[1]
Whereas there were no reported adverse events related to the use of the counterfeit product, it nonetheless sounded the alarm over fake HIV medications potentially reaching medicine cabinets in American households. Fortunately, Janssen's strong ties to the patient advocacy community helped to raise awareness quickly to counter the threat (Editor's Note: The ADAP Advocacy Association at the time pushed numerous Tweets about the alert).
The most recent threat wasn't the first incident whereby fake HIV medication had found its way into the nation's drug supply chain. In 2001, counterfeit Serostim® – prescribed injectable medicine used for the treatment of HIV-related wasting – was found in U.S. pharmacies nationwide. According to news reports at the time, Serono, Inc. countered the threat by mailing drug alert letters to distributors, pharmacies, physicians and patients, as well as sending hundreds of alerts to AIDS service organizations.[2]
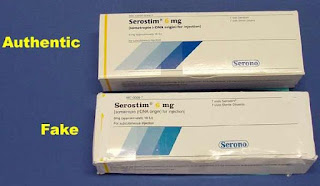 |
| Photo Source: Willis QS Consulting |
The Partnership for Safe Medicines (PSM), which is the preeminent policy expert on counterfeit drugs, has raised awareness about fake insulin, fake cancer medications, and fake hepatitis medicines. In the past, PSM has also tackled fake HIV medications.
Rick Roberts, an HIV-positive advocate on the PSM board of directors, has spent the past two decades warning of the threat of counterfeits. Roberts argues, "For all-in-one treatments, the possibility that a counterfeit could leave a patient without any treatment at all is a heartbreaking life-threatening situation. Having counterfeit Symtuza show up in a licensed U.S. pharmacy, as the counterfeit Serostim I received in 2000 did, is proof that our supply chain can't allow any foreign wholesalers or pharmacies that can't easily be regulated as some proposals to do foreign drug importation suggest.
According to Roberts, at least the counterfeiters that targeted him could be brought into court. Many foreign counterfeit criminals never see the inside of the U.S. courtroom.
Counterfeit medication isn't something that only happens in "other" countries, evidenced by warnings on the FDA website. Although the U.S. drug supply chain remains among the safest and most secure systems internationally, it still remains susceptible to nefarious activities. Fake HIV medications, such as Symtuza or Serostim, should serve as a reminder to patients and policy-makers alike that more needs to be done to protect our access to medications.
Disclaimer: Guest blogs do not necessarily reflect the views of the ADAP Advocacy Association, but rather they provide a neutral platform whereby the author serves to promote open, honest discussion about public health-related issues and updates.
[2] Naomi Aoki (2001, February 5). Maker of AIDS Drug Battles Counterfeiters / Serostim's growth hormone may give it black-market value. Boston Globe. Retrieved online at https://www.sfgate.com/business/article/Maker-of-AIDS-Drug-Battles-Counterfeiters-2955169.php.

No comments:
Post a Comment