By: Ranier Simons, ADAP Blog Guest Contributor
Ideological attacks on evidence-based established medical science through litigation continue to threaten the healthcare of millions. New legal challenges from anti-abortion groups restricting access to mifepristone join continued pushback from the Braidwood vs. Becerra decisions in the current news cycle.
In November of 2022, an anti-abortion coalition of doctors called The Alliance for Hippocratic Medicine sued the U.S. Food and Drug Administration (FDA) stating that the agency improperly approved the drug mifepristone in September 2000. The group purports that the drug has serious safety issues that the FDA did not adequately consider. It claims the studies the FDA cites confirming the safety of mifepristone are flawed; thus, the drug should be taken off the market and approval revoked. Additionally, the group claims the FDA approved the drug using an accelerated process that was not thorough, thus abusing its authority.[1]
 |
| Photo Source: Ohio Capital Journal |
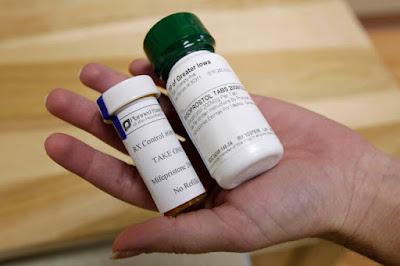
Mifepristone used in conjunction with misoprostol is the current established method of chemically inducing the termination of a pregnancy. Mifepristone is a steroid that starts the process and misoprostol makes the body expel the pregnancy tissue. Data has proven the duo to be the most effective with the least number of complications. Using misoprostol alone is not as effective and can result in complications that result in the need for surgical intervention to complete the abortion. It is safe to use misprostol alone with 93% of women using it alone having complete abortions without surgical assistance.[2] However, there are currently no official FDA backed protocols for using it alone.
Attorneys from the Alliance Defending Freedom represent the physicians. Alliance Defending Freedom is the same group that worked with Mississippi lawmakers on the suit at the center of the Dobbs v. Jackson Women’s Health Organization decision, which eventually overturned Roe V. Wade.[1] The group made efforts to get the case heard by Judge Matthew Kacsmaryk, of the U.S. Northern District of Texas, a judge Trump appointed in 2019.
The Amarillo division of the Northern District of Texas is a federal district with a single judge, Judge Kacsmaryk. The attorneys knew of his anti-abortion history, which is why they filed in his district. Judge Kacsmaryk has also issued rulings against protections for transgender persons and against asylum seekers forcing a return to Mexico while awaiting processing.[3] Moreover, Judge Kacsmaryk concealed from the Senate Judiciary Committee his authoring of an article titled “The Jurisprudence of the Body,” published in September 2017 in the Texas Review of Law and Politics.[4] In the article, he stated the Obama Administration had ignored the beliefs of religious physicians who “cannot use their scalpels to make female what God created male” and “cannot use their pens to prescribe or dispense abortifacient drugs designed to kill unborn children.”[4]
The case before Judge Kacsmaryk has not gone to trial yet. However, he held a hearing regarding the issue, whose results were released on April 7, 2023. He stated that the Alliance for Hippocratic Medicine had legal standing to sue, and that the FDA’s approval of mifepristone should be suspended nationwide while the case plays out in court. Additionally, he delayed the effective date of his ruling for a week to allow the Biden Administration to appeal.[1] The decision was appealed to the United States Court of Appeals for the Fifth Circuit, which ruled partially against Judge Kacsmaryk on April 12, 2023.
The appeals court ruled that Judge Kacsmaryk could not reverse the FDA’s 2000 approval of mifepristone because the statute of limitations for the plaintiffs to claim harm had passed. However, the three-person appeals panel added restrictions. The restrictions effectively reversed additional approvals for use the FDA issued in 2016 based on ongoing evidence-based research that had developed since its initial approval. The appeals court ruled that mifepristone should only be used up to 7 weeks of pregnancy, down from its current limit of 10 weeks. Additionally, it ruled that the drug could no longer be sent through the mail, it could only be obtained in person via three different doctor visits, and it could only be administered by a qualified physician instead of other medically qualified and licensed caregivers. The appeals court also invalidated the FDA’s 2019 approval of a mifepristone generic made by GenBioPro.
This ruling still greatly restricts mifepristone’s usage. It prevents pharmacies, both in-person and by mail, from dispensing the medication. Shortening the window in which the drug can be used reduces options for women depending on when they discover they are pregnant. The requirement of in-person doctor visits is one of the most significant hindrances. Requiring in-person doctor visits increases patients' costs of paying for multiple doctor visits, whether through insurance copay cost sharing or entirely out of pocket if uninsured. Additionally, it prohibits prescribing the drug through telehealth visits.
 |
| Photo Source: WHYY PBS | The Associated Press |
In response to the appeal court’s ruling, the U.S. Department of Justice (DOJ) submitted an emergency consideration before the Supreme Court. As a result, on April 14, 2023, U.S. Supreme Court Justice Samuel Alito issued a temporary stay on all the lower court rulings. The temporary stay leaves mifepristone usage unchanged from the status quo until 11:59 PM Wednesday, April 19, 2023.[5] Both sides are instructed to submit responses so that the Supreme Court can ultimately rule if mifepristone can remain unencumbered while the appeal plays out or if the appeal court limits will be reinstated pending court trial.
U.S. Solicitor General Elizabeth Prelogar stated that “the lower court rulings are the first-time judges have repealed the conditions of an FDA drug approval based on a disagreement over the agency’s judgment about safety.”[5] Many pharmaceutical companies, patient advocacy groups, physicians, and government officials have been sounding the alarm concerning the danger of the initial case and its appeal. On April 14, 2023, 253 members of Congress (50 Senators and 203 Representatives) filed an amicus brief asking the Supreme Court to give relief from the district court ruling and the appellate ruling.
They stated in the brief that “emergency relief from the order is necessary to mitigate the imminent harm facing members of the public, many of whom rely on the availability of mifepristone for reproductive care—and many more of whom rely on the integrity of FDA’s drug approval process for continued access to life-improving and life-saving drugs. Congress intended to—and did—vest authority in FDA to evaluate and ensure the safety and efficacy of drugs in the United States, and Amici call on this Court to give due weight to that intent.”[6]
A group of over 160 CEOs, healthcare organization presidents, and physicians also filed an amicus brief detailing the negative consequences of the ruling from an industry perspective.[7] In the brief, they explain several arguments against Judge Kacsmaryk’s and the appeals court ruling. The central idea is that the judge nor the court has the scientific or medical expertise to challenge the validity of the evidence behind the FDA. Mifepristone has been in use for over 20 years and has been extensively researched. The lower court rulings would also prevent the FDA from effectively using new data that comes from comparative safety data from real-world evidence. As drugs are used, continuous examination results in changes to initial use recommendations. The lower court rulings stifle the FDA’s ability to depart and evolve from a drug’s initial clinical trial findings.
The legitimacy of the authority of the FDA is what is at stake. Rachel King, BIO's Interim President and CEO, stated: “We’ve repeatedly warned that the District Court’s decision would set a dangerous precedent for undermining the FDA, has consequences that extend well beyond the single drug, and stokes regulatory uncertainty in an industry responsible for bringing life-saving and life-enhancing therapies to vulnerable patient populations.”[8]
Allowing ideology and special interest agendas to upend medical science threatens public health. It also threatens destabilization of the entire drug development infrastructure and investment. The results of the cases will have ripple effects regardless of the resolution.
[1] Kimball, S., Luhn, M., Mangan, D. (2023, April 7). Federal judge suspends FDA abortion pill approval, gives Biden administration time to appeal. Retrieved from https://www.cnbc.com/2023/04/07/federal-judge-stays-fda-abortion-pill-approval-gives-time-to-appeal.html
[2] University of California San Francisco. (2023, February 26). The recommended protocol for misoprostol-Only abortion. Retrieved from https://www.ansirh.org/research/research/recommended-protocol-misoprostol-only-abortion
[3] Kitchener, C., Marimow, A. (2023, February 25). The Texas judge who could take down the abortion pill. Retrieved from https://www.washingtonpost.com/politics/2023/02/25/texas-judge-abortion-pill-decision/
[4] Kitchener, C., Marimow, A., Barnes, R. (2023, April 15). The controversial article Texas federal judge Matthew Kacsmaryk did not disclose to the Senate. Retrieved from https://www.texastribune.org/2023/04/15/kacsmaryk-law-review-article-washington-post/
[5] Kimball, S. (2023, April 14). Supreme Court lifts abortion pill restrictions for now. Retrieved from https://www.cnbc.com/2023/04/14/supreme-court-temporarily-blocks-abortion-pill-restrictions.html
[6] Murray, P. (2023, April 14). Democrats in Congress File Amicus Brief Urging Supreme Court to Prevent Dangerous Ruling From Restricting Access to Mifepristone Nationwide & Upending FDA Approval Process. Retrieved from https://www.murray.senate.gov/democrats-in-congress-file-amicus-brief-urging-supreme-court-to-prevent-dangerous-ruling-from-restricting-access-to-mifepristone-nationwide-upending-fda-approval-process/[8
[7] Case 23-10362. (2023, April 11). Unopposed motion for leave to file brief of pharmaceutical companies, executives, and investors as amici curiae in support of appellants' motion for stay pending appeal. Retrieved from https://www.bio.org/sites/default/files/2023-04/2023-04-11-amicus-brief.pdf
[8] BIO. (2023, April 11). Press Release: BIO joins amicus brief challenging court's efforts to undermine FDA's authority to bring treatments and cures to patients. Retrieved from https://www.bio.org/press-release/bio-joins-amicus-brief-challenging-courts-efforts-undermine-fdas-authority-bring
Disclaimer: Guest blogs do not necessarily reflect the views of the ADAP Advocacy Association, but rather they provide a neutral platform whereby the author serves to promote open, honest discussion about public health-related issues and updates.

No comments:
Post a Comment