By: Ranier Simons, ADAP Blog Guest Contributor
On Saturday, July 23, 2022, the World Health Organization (WHO) declared monkeypox a PHEIC, public health emergency of international concern.[1] Internationally, the number of cases has reached over 16,000 in over 75 European countries, North and South America, the Middle East, South Asia, Australia, and other parts of Africa not previously exposed.[1] In the United States, there have been roughly 2,900 cases.
It is important to note that anyone can contract monkeypox. Monkeypox was first identified in 1958 in a colony of research monkeys.[2] Since 1970 human outbreaks have been reported in 11 African countries. The first outbreak outside of Africa was in 2003 in the United States. It occurred in several midwestern states and was linked to infected prairie dogs that people had as pets. Those pets likely contracted the disease from being housed with infected rats and door mice shipped from Ghana.
 |
| Photo Source: National Institute for Communicable Diseases |
Although anyone can contract the disease, the recent outbreak disproportionately affects gay, bisexual, and men who have sex with men (MSM).[2] The U.S. Centers for Disease Control & Prevention (CDC), the media, and health professionals have consciously not labeled monkeypox a gay disease. It indeed is not a gay disease. However, given that the current outbreak mainly affects gay men, it is essential to clarify that explicitly. The award-winning blogger Mark S. King points out, "Gay men are getting monkeypox and suffering greatly. When gay men understand the threat, we are more likely to take precautions, get vaccinated, or be informed about treatment”.[3]
Concerns about stigma are valid. Gay men historically have and continue to face stigma and apathy in the healthcare arena and public opinion regarding healthcare issues. But, King also points out that it is dangerous to bury facts with vague and evasive messaging.[3] Vague messaging leads the general public to think that their present odds of contracting the disease are higher than the reality of the numbers. Conversely, labeling monkeypox a gay illness would make the public complacent about being mindful of their activities and being tested when they should be.
The Washington Post’s Benjamin Ryan points out that “…public health experts know well, epidemiology is less concerned with whether someone could contract an infection; instead, the much more vital questions focus on which groups of people are most likely to be exposed to a pathogen, to contract it and why.”[4] There need to be targeted education and prevention efforts aimed at gay men to emphasize the specifics that make them more susceptible to the spread of monkeypox. Facts show that the sexual and social networks of gay men are why it is hitting the population hard. There have been clusters of infections traced back to events such as large circuit parties, nightclub events, and pool parties. Bathhouses are the settings of some clustered outbreaks, as well. Gay men need to be informed of the statistical fact of gay men having an increased incidence of multiple sex partners, especially in combination with certain events and travel, which also increases the likelihood of the spread of monkeypox amidst their demographic.
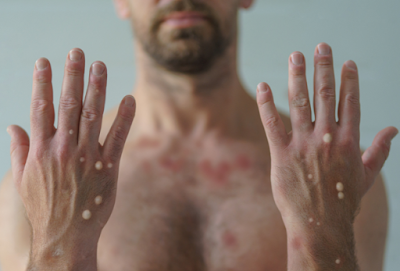
Monkeypox is contracted through close skin-to-skin contact. Transmission comes from exposure to broken skin, mucus membranes, respiratory droplets, infected bodily fluids, and even contact with contaminated linens. It is not airborne like Covid-19. Rubbing, kissing, and physical contact are enough to cause infection. Sexual intercourse is not necessary. Seven to fourteen days after infection is when symptoms usually appear. Fever, chills, exhaustion, headaches, and muscle weakness are typically initial signs.[2] Progression includes the swelling of lymph nodes and widespread body rash that can include the mouth, hands, and feet. Then fluid-filled painful pox can appear on the body, surrounded by red circles. There is increased reporting that some infected individuals only see some rash and painful pox sores in the genital and anal regions instead of all over the body.
 |
| Photo Source: PBS |
Over 190,000 doses of the two-dose Jynneos vaccine have been pulled from the Strategic National Stockpile. Jynneos is used for the prevention of monkeypox and smallpox. There is still not enough in circulation to meet the demand for vaccination nor to vaccinate all those in the highest risk categories. There is also an antiretroviral effective against monkeypox named TPOXX (tecovirimat), approved by the U.S. Food & Drug Administration (FDA) for use against smallpox in 2018.[5] Unfortunately, TPOXX was only authorized for smallpox because it is deadly and can be considered a possible bioterrorism weapon. Monkeypox is not fatal like smallpox. Thus obtaining TPOXX requires many pages of paperwork to get it from the Strategic National Stockpile. Additionally, protocol requires doctors to submit pictures of a patient's lesions to the local health department or CDC and a folio of pages of detailed information to get TPOXX. After getting the drug, patients are required to keep a daily journal while they are taking it.[5]
Presently, monkeypox prevention is paramount as vaccination and treatment options are scarce. Effectively educating gay, bisexual, and men who have sex with men with the unadulterated details of their demographics’ statistically documented behaviors put them at high risk is the best weapon against the spread of the disease. Targeted explicit information delivered with respect and dignity is the best way to avoid stigma while undergirding the importance of the information.
[1] Branswell, H. (2022, July 23). WHO declares monkeypox outbreak a public health emergency. Retrieved from https://www.statnews.com/2022/07/23/who-declares-monkeypox-outbreak-a-public-health-emergency/
[2] Howard, J. (2022, July 21). Monkeypox spreading in 'cluster events,' but vaccines can help stop it, local health officials say. Retrieved from https://www.cnn.com/2022/07/21/health/monkeypox-clusters-local-officials/index.html
[3] King, M. (2022, July 19). Monkeypox is a gay thing. We must say it. Retrieved from https://marksking.com/my-fabulous-disease/monkeypox-is-a-gay-thing-we-must-say-it/
[4] Ryan, B. (2022, July 18). You are being misled about monkeypox. Retrieved from https://www.washingtonpost.com/opinions/2022/07/18/monkeypox-gay-men-deserve-unvarnished-truth/
[5] Walsh, D. (2022, July 21). There Is a monkeypox antiviral. But try getting it. Retrieved from https://nymag.com/intelligencer/2022/07/tpoxx-is-a-monkeypox-antiviral-but-try-getting-it.html
Disclaimer: Guest blogs do not necessarily reflect the views of the ADAP Advocacy Association, but rather they provide a neutral platform whereby the author serves to promote open, honest discussion about public health-related issues and updates.





