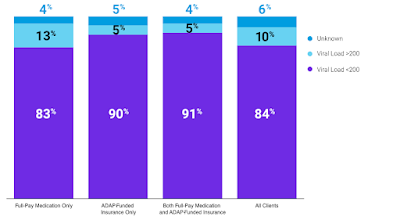
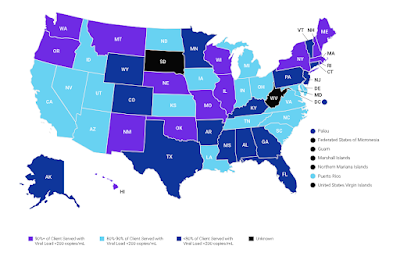
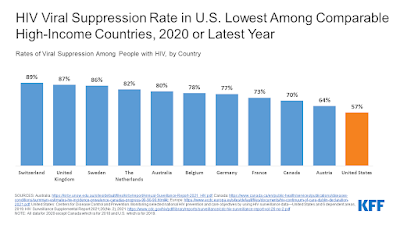
By: Ranier Simons, ADAP Blog Guest Contributor
The increasing consolidation of corporate health entities is of significant concern because of the vast number of lives affected. When companies control a substantial market share of services and products, every business practice has pervasive outcomes since so many end users are dependent on them. This is especially notable in the arena of prescription drug benefit management, where CVS Health is one of the most prominent players. CVS Health has a history of actions and practices that have proven to be problematic in the lives of many patients and consumers. Moreover, their practices have habitually adversely affected people living with HIV/AIDS (PLWHA). Recently, CVS unsuccessfully tried to evade a discrimination lawsuit due to their practices.
 |
| Photo Source: TheBody |
In 2018, seven plaintiffs filed a class action lawsuit against CVS Health Corporation, claiming CVS discriminated against PLWHA based on their prescription plans' design and denying them meaningful access to their health benefits.[1] The employer-sponsored health plan the patients utilized, whose pharmacy benefits were managed by CVS, required them to use mail-order specialty pharmacy services or CVS chain pharmacy services to obtain their HIV medication and receive the in-network pricing. They could not use their preferred in-network community pharmacies without incurring significantly higher out-of-pocket costs.
Utilizing preferred in-network pharmacies results in optimal patient care and utilization of services. Local pharmacies allow face-to-face interactions with pharmacists familiar with patients’ medical histories and related needs. Additionally, they are often geographically more accessible than the CVS chain pharmacies. Mail-order dispensing can result in delayed medication, lost or stolen medication, damage to medicines due to exposure to the elements, and even privacy concerns.
Repeatedly, the plaintiffs petitioned both their employer and CVS to ‘opt-out’ to be able to use their preferred in-network pharmacy choices, which were considered in-network for non-HIV medications. Despite repeated communications and requests, neither CVS nor their employers allowed them to opt out.[1] As such, they brought the lawsuit alleging that CVS was engaged in discrimination based on HIV disability under the Affordable Care Act. CVS moved to have the case dismissed on several grounds.[2] Firstly, they argued that the case had no merit because several of the plaintiffs are now deceased. They also argued the case should be dismissed because a couple of plaintiffs are now on different plans not currently associated with the problematic CVS benefit issue in question. Most notably, they claimed that they were not aware that their policies directly affected the rights of a federally protected class. Additionally, they argued that the employers were responsible for not allowing the plaintiffs to opt-out, not CVS.
 |
| Photo Source: Chronic Disease Coalition |
In April 2024, U.S. District Judge Edward Chen ruled that CVS’ request for dismissal would not be granted.[2] He ruled that CVS’s actions fulfilled the requirements of proving deliberate indifference. The legal term means that CVS was fully aware of the damage the benefit plan specifically had on the PLWHA’s medically necessary access to HIV medications but failed to act on it by making reasonable accommodations.[2] In addition to the well-documented communications between the plaintiffs and CVS, CVS internal reports indicated that CVS had been given previous legal guidance that their benefit design could be deemed discriminatory to a protected class.
Judge Chen also ruled that CVS could not blame the employer. CVS was fully able to modify a plan structure whenever it wanted. Moreover, the plans CVS presented to employers to select for coverage contained financial incentives for forcing the use of mail-order and CVS-specific branded pharmacies for HIV medication and other specialty drugs.[2] It was proven that CVS had other plans that allowed patients to opt out but did not make those options available for the employers in question. Unfortunately, Judge Chen ruled against the plaintiffs, stating they could not sue for monetary damages from the thousands of dollars they had paid out of pocket to get their medications from their preferred community pharmacies. Regardless, the plaintiffs legally have standing for the case to proceed because of Chen’s denial of the dismissal.
As a result, CVS was sued in a separate lawsuit in 2018 for errantly publicly disclosing the HIV status of over 6,000 Ohio residents.[3] The Ohio AIDS Drug Assistance Program (OhDAP) had a contract with CVS where CVS provided HIV medications to OhDAP clients and handled communications with those members. The suit alleged that in the third quarter of 2017, CVS mailed out a letter containing membership cards, information about how to obtain HIV medications, and other program information to 6,000 clients. This mailing was in an envelope with a clear plastic window with a short reference code for the mailing list, ‘PM 6402 HIV’ printed above the recipient’s name. The plaintiffs sought legal remedy because the mailer "resulted in the potential or actual disclosure of recipients' HIV status to numerous individuals, including their families, friends, roommates, landlords, neighbors, mail carriers, and complete strangers."[4]
 |
| Photo Source: KRSO |
The mailing was not only negligent in its handling of protected health information but was seemingly profit-driven. At the time of the mailing, many of the recipients did not have an established relationship with CVS. The mailer was sent regardless of whether the recipients were active CVS pharmacy customers. It was a way to market the usage of not only CVS pharmacy benefit products but also its general health care delivery services.[5] In 2020, CVS agreed to settle the lawsuit for $4.35 million.[6]
CVS is not the only problematic player in the corporate healthcare arena. However, their actions and core paradigms are consistently antagonistic to the best interests of PLWHA and others utilizing specialty drugs. In a briefing presented to payors in 2021, CVS Caremark listed strategies it would employ to save money. Regarding activities in response to new drugs in the market, some of its planned actions included: initially blocking coverage of new drugs, reviewing for clinical appropriateness and cost-effectiveness prior to formulary decision, applying aggressive clinically appropriate utilization management criteria with rigorous approval requirements, strongly favoring generic use requiring objective evidence of need for newer agents, and selecting preferred agents generating lowest net cost option in each category.[7]
Jen Laws, CEO of Community Access National Network, summarily describes CVS, stating: “CVS engages in aggressive self-dealing practices and anti-competitive contract efforts to eliminate competition, drive patients to their own pharmacies (including by way of under reimbursement to independent, non-chain pharmacies and higher cost-sharing for patients utilizing their trusted pharmacies), and roundly limit access to care by weaponizing their PBM.” It seems that keeping a continued focus on CVS will be necessary to highlight and act against present and future practices that harm patients.
[1] Third Amended Class Action Complaint. (2023, October 24) ). Retrieved from https://consumerwatchdog.org/wp-content/uploads/2023/11/2023-10-24-241_Third-Amended-Complaint.pdf
[2] John Doe One et al. v. CVS Pharmacy Ruling. (2024, April 18). Retrieved from https://www.courthousenews.com/cvs-medication-program-discriminates-against-hiv-aids-patients-judge-says/john-doe-one-et-al-v-cvs-pharmacy-et-al-mtd-ruling/
[3] Doe One et al. v. CVS Health Corporation et al. (2018, March 21). Retrieved from https://dockets.justia.com/docket/ohio/ohsdce/2:2018cv00238/211764
[4] Faul, A. (2018, March 29). CVS Health unintentionally revealed HIV status of 6,000 customers: Lawsuit. Retrieved fromhttps://abcnews.go.com/Health/cvs-health-unintentionally-revealed-hiv-status-6000-customers/story?id=54095674
[5] Schladen, M. (2018, June 29). State, CVS sued over HIV mailing. Retrieved from https://www.dispatch.com/story/news/politics/elections/2018/06/29/state-cvs-sued-over-hiv/11624806007/
[6] AIMED Alliance. (2020, May 15). CVS Health to Settle Lawsuit for Revealing HIV Status of Over 4,500 Patients. Retrieved fromhttps://aimedalliance.org/cvs-health-to-settle-lawsuit-for-revealing-hiv-status-of-over-4500-patients/
[7] Accetta, L. (2021). Expanding therapies, indications and implications for payors: Pipeline trends that will drive change in 2022. Retrieved from https://business.caremark.com/insights/2021/expanding-therapies-indications-and-implications-payors.html
Disclaimer: Guest blogs do not necessarily reflect the views of the ADAP Advocacy Association, but rather they provide a neutral platform whereby the author serves to promote open, honest discussion about public health-related issues and updates.