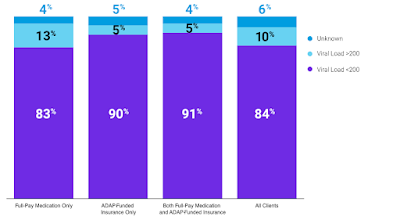
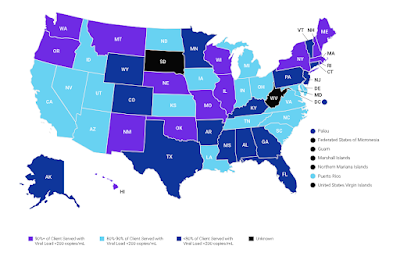
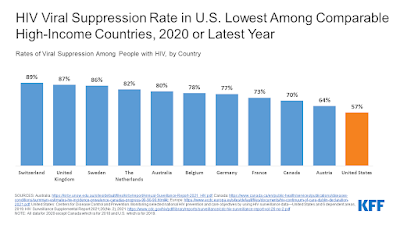
By: Ranier Simons, ADAP Blog Guest Contributor
Numbers may be boring, but when it comes to matters of life and death, numbers are essential, and quality data is indispensable. The Centers for Disease Control and Prevention (CDC) last week released its most recent HIV Incidence and Prevalence report.[1,2] The report is based on data from 2018-2022, with the data gathering completed in December 2023. The U.S. is not currently on track to reach Ending the HIV Epidemic (EHE) 2025 and 2030 prevention goals.[3] While progress is being made, there remains much work that needs to be done.
 |
| Photo Source: HIV.gov |
Overall, estimated new HIV infection rates declined 12% in 2022 in comparison to 2018 among people 13 years of age or older.[2] The overall rate of decline encompasses higher levels of decline of specific groups. Bear in mind that the 12% decrease is attributed to a decline among men. No statistically significant change was observed among females. It is important to note the data is based on the sex assigned at birth (SAAS).[2] Although there was an overall decrease in the rate of new HIV infections, it is important to note that in 2022, 81% of all new infections were among males.[2]
A notable decrease, giving optimism for future trends occurred among young people. The largest reduction in HIV infections in 2022 compared to 2028 was among men ages 13-24, at 30%. The decrease was predominately among gay and bisexual men. The CDC suggests the data shows efforts to reach that demographic are working regarding testing, treatment, and PrEP uptake.
Concerning race and ethnicity, the new report indicated an 18% decrease in the HIV infection rate among people identifying as Black/African American.[2] In 2022, 37% of all new infections were among Black/African American persons, and the highest rate of infection was observed among the same group. The 18% decrease is undoubtedly a positive progression. However, people identifying as Black/African American are only 12% of the general population. Thus, the high infection rate informs the existence of factors causing disparities that need to be examined and corrected. The highest rates of new infection in 2022 in the US (per 100,000 people) were Black/African Americans at 34.1, those identifying as multiracial at 21.6, and Hispanic/Latino persons at 20.7.[2]
 |
| Photo Source: CDC |
Regionally, data indicates a welcome improved trend in the South. Even though 49% of new infections in 2022 were in the South, the region experienced a 16% decrease in new infection rate.[2] Regional rates were 14.5 South, 11.0 West, 8.9 Northeast, and 7.4 Midwest.[2] The South historically has a heavy burden of HIV in comparison to the rest of the country. Much of that has been driven by socioeconomic factors. The region has the highest poverty rate and lowest household median income.[2,5] The South is also characterized as having the highest knowledge gap of HIV status in the US. In 2022, the report indicates that 14 out of 100 people 13 years of age and older in the South were unaware of their HIV status.[2] Lack of knowledge of status could mean untimely receipt of medical care and treatment.
The category of transmission was another positive area showing a decrease. There was a 10% decrease in new infections attributed to male-to-male sexual contact (MMSC) and a 27% decrease when grouping together MMSC and injection drug use (IDU).[2] There were no changes detected regarding heterosexual contact and IDU, nor infections among females. In 2022, 67% of all new HIV infections were a result of MMSC. Among male new infections, 83% were attributed to MMSC. Thus, the 10% decrease is a step in the new direction.
These trends are just a few of the positive highlights revealed in the latest CDC HIV surveillance report. While positive, statistics show that the US is not on track with EHE goals and lags improvements made in other developed countries. Hopefully, this new data will continue to drive optimism as well as drive continued scientific, financial, legislative, and social bolstering of the multifaceted fight to eradicate HIV.
[1] CDC. (2024, May 21). Estimated HIV Incidence and Prevalence. Retrieved from https://www.cdc.gov/hiv-data/nhss/estimated-hiv-incidence-and-prevalence.html?CDC_AAref_Val=https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
[2] CDC. (2024, May 21). HIV Surveillance Supplemental Report: Estimated HIV Incidence and Prevalence in the United States, 2018–2022. Retrieved from https://stacks.cdc.gov/view/cdc/156513.
[3] CDC. (2024, March 30). Ending the HIV Epidemic in the US Goals. Retrieved from https://www.cdc.gov/ehe/php/about/goals.html
[4] Carson-Holt, E. (2024, May 2024). HIV transmissions down by 12%. One age group in particular is bringing those numbers down. Retrieved from https://www.lgbtqnation.com/2024/05/hiv-transmissions-down-by-12-this-age-group-in-particular-is-bringing-those-numbers-down/
[5] AIDSVU. (2024). Deeper Look: HIV in the South. Retrieved from https://aidsvu.org/resources/deeper-look-south/#:~:text=The%20heavy%20burden%20of%20HIV,factors%20like%20poverty%20and%20unemployment.
Disclaimer: Guest blogs do not necessarily reflect the views of the ADAP Advocacy Association, but rather they provide a neutral platform whereby the author serves to promote open, honest discussion about public health-related issues and updates.